Cell fate control in development and disease
The aim of our lab's research is to understand how cells acquire their fates during development and how these processes go wrong in congenital disease.
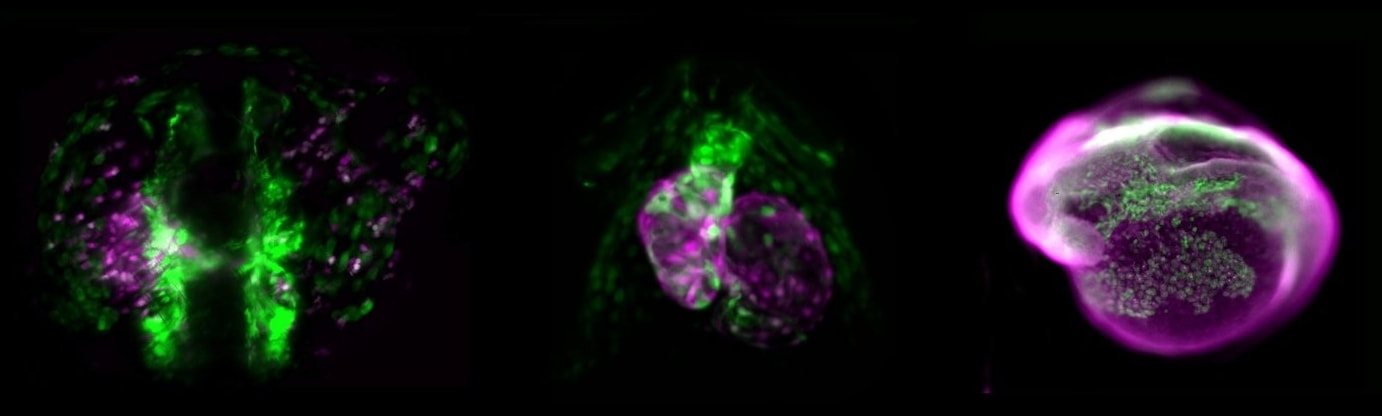
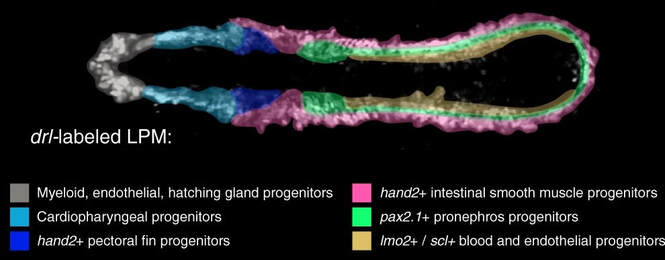
As principal model, we use the zebrafish (Danio rerio) to investigate the cell fate control of mesodermal lineages, in particular of the lateral plate mesoderm (LPM). The LPM harbors echos of an ancestral body plan dating back millions of years that continues to help us understand how our body and organs develop today. Our work combines transgenics, genome editing, cross-species analysis of cis-regulatory elements, and lightsheet/SPIM-based live imaging. We further work with chicken embryos and collaborate with a diverse group of international collaborators to integrate a variety of chordate model systems into our research.
Key questions we are pursuing:
1) What is the developmental and evolutionary origin of the cardiovascular system?
2) How can the LPM form its vast spectrum of downstream cell fates?
3) What mechanisms result in congenital defects of LPM-derived organ systems, such as congenital heart disease?
In addition, we continue to develop and expand in vivo techniques for lineage tracing, in particular using the Cre/lox system and photoconvertible fluorophores combined with lightsheet imaging in zebrafish. We generate transgenics using Tol2 and our new safe-harbor landing sites using the pIGLET system!

We are active members of the Zebrafish Disease Models Society (ZDMS), with Christian Mosimann serving on the Research Interest Group on Cardiac and Muscle Diseases. ZDMS provides a platform for research groups working on models of human disease using the zebrafish, with an annual meeting and growing international community.